A Strong Pathway to Regulatory Submission
North American Science Associates (NAMSA) is the world’s only 100% medical device-focused, full continuum Contract Research Organization. Driven by its global regulatory expertise and in-depth therapeutic knowledge, NAMSA is dedicated to accelerating medical device product development efficiently and cost-effectively. MediCarbone has partnered with NAMSA to receive guidance on the regulatory pathway, evidence requirements, and pre-clinical and clinical testing plans necessary for FDA regulatory submissions.
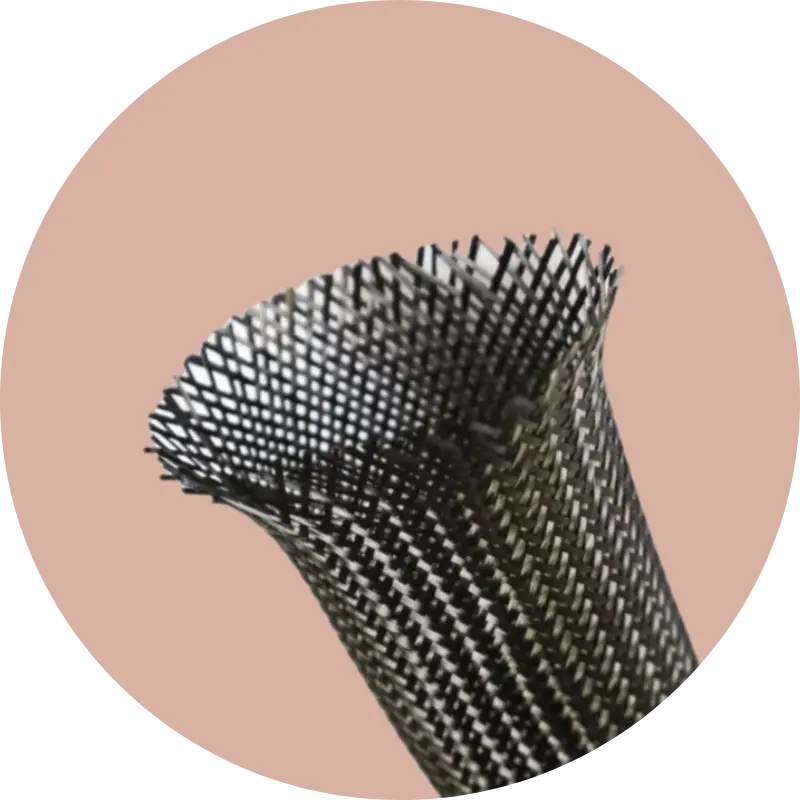
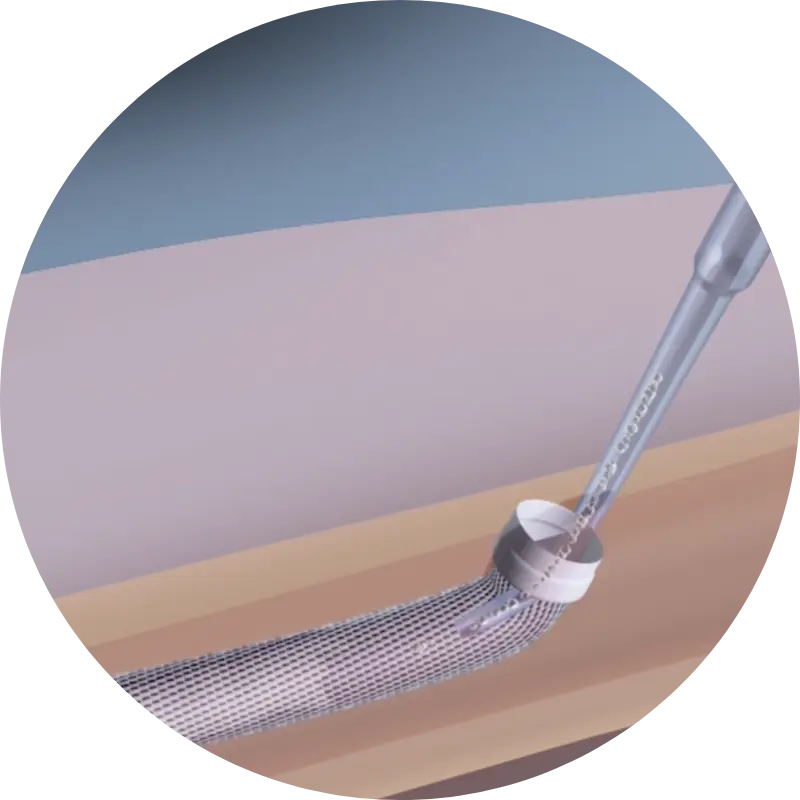
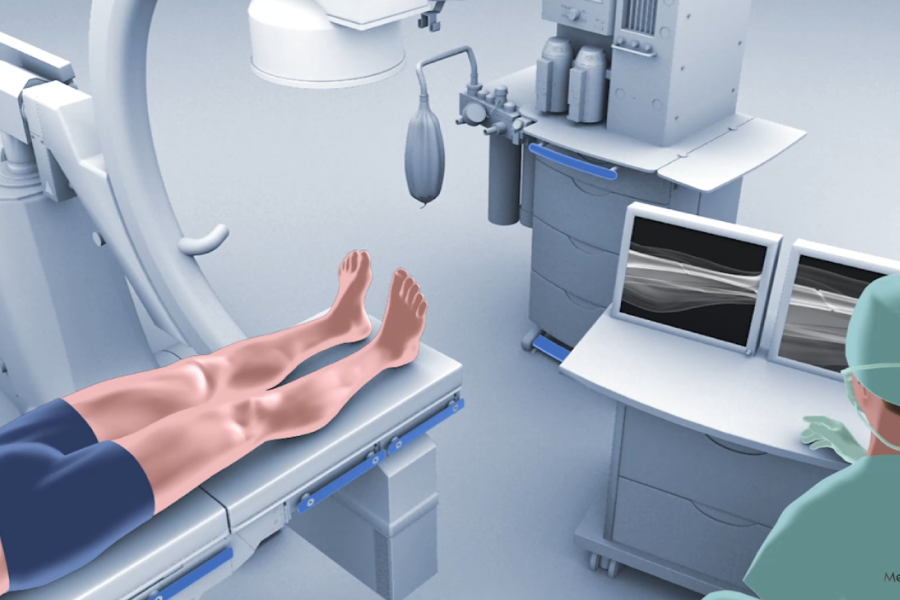
Born from a premier research university, backed by a proven team, and bolstered by patented technology and FDA-approved materials—we seek to revolutionize bone fracture repair through minimally invasive, customized intramedullary nailing technology.
Latest News
See us at the Orthopedic Research Society Annual Meeting (ORS)
MediCarbone will be in attendance at the Orthopedic Research Society Annual Meeting (ORS), February 2-6, 2024 at the
MediCarbone patent information
MediCarbone has 1 licensed patent from the University of Arizona; 2 patents approved for publishing and many
MediCarbone receives NSF SBIR Phase I funding
MediCarbone announced it received an NSF SBIR Phase I award for $273,563 for the design and development of